All You Need Is Air™
Generate Continuous, Unlimited iNO at the Point of Care.
LungFit® PH uses patented Plasma Pulse Technology™ to generate inhaled nitric oxide (iNO) from ambient air in the room, eliminating the need for iNO source supplies like cylinders or cassettes.1,2
Initiate iNO therapy quickly and simply
- Start iNO therapy in 1 minute with quick, simple steps1,2
- Power on, make 3 familiar connections, and set a dose
- Unburden your respiratory therapy (RT) department and enable more time to focus on patient care3
- No concerns about watching a supply source, such as cylinders or liquid-filled cassettes, because you never run out of room air
- Predictable 12-hour filter life regardless of dose or gas flow
- Can coordinate filter changes with vent checks for efficiency


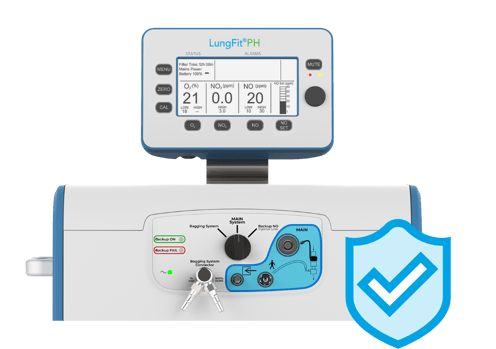
Confidently deliver the set dose
- NO delivery of 0.1–80 ppm, gas flow of 0.5–100 L/min
- Main and backup generators are the size of a D battery and use the energy of a 60W bulb to produce NO from room air
- Main, backup, and bagging modes with the flexibility to deliver iNO continuously through both the main injector line and bagging system
- Proven dual-channel independent delivery and monitoring methods for confidence in delivering a set dose
- Precise real-time measurement of ventilator gas flow to maintain accurate and stable iNO delivery
- Up to 4 hours backup battery life
- Empower staff with limited iNO experience to be confident delivering nitric oxide therapy

Enhance efficiency by eliminating need for iNO source supplies
- Eliminate burden of inventory management, storage space, and safety risk for iNO supplies
- No special storage requirements
- No hazardous materials or heavy, bulky cylinders
- No storage space needed for full and depleted cylinders
- All other components are disposable patient accessories
- No reusable components requiring sterilization
Request a call to explore your cost savings.
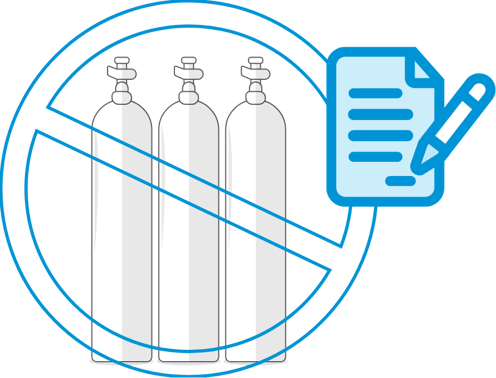
Discover the simplicity of iNO therapy without cylinders or cassettes.3
Exclusive Plasma Pulse Technology™
The first and only FDA approved system that generates iNO from room air.1
Here’s how it works:
For Illustrative Purposes
Compact and energy-efficient: Generators the size of a D battery use minimal power to reliably produce iNO from room air
Micro-pulses of energy produce a precise amount of NO to deliver the set dose
NO2 Smart Filter removes NO2 from the internal circuit to reduce patient and staff exposure
Exceeding your expectations with:

(technical, clinical, commercial)

Emergency deliveries within 6 hours*

**LungFlex Rapid Replacement response times are based on hospital locations. While every effort is made to accommodate emergency deliveries within 6 hours of request, some hospital locations may take longer.
Please contact Beyond Air Medical Information regarding any medical questions: medinfo@beyondair.net
Explore your cost savings and find out how you can get annual budget certainty at a competitive price
 All-inclusive, contracts including device, accessories, and 24/7 service and support
All-inclusive, contracts including device, accessories, and 24/7 service and support
 Custom-built proposals based on device needs and volume of usage
Custom-built proposals based on device needs and volume of usage
Experience iNO without cylinders or cassettes.
Complete the form below to schedule a demo or call.

References: 1. LungFit PH System Operator’s Manual. Garden City, NY: Beyond Air Inc. 2022. 2. Data on file. Beyond Air Inc. 2021. 3. Rimkus M, Bathe D, Montgomery F. On-demand nitric oxide for ventilator-based nitric oxide inhalation: a risk-reduction perspective. Abstracts of International Society for Aerosols in Medicine e.V. 22nd ISAM Congress, May 24, 2019, Montreux, Switzerland. J Aerosol Med Pulm Drug Deliv. 2019;32(3). https://doi.org/10.1089/jamp.2019.ab02.abstracts
Important Safety Information
INDICATIONS FOR USE
The nitric oxide from the LungFit PH System is indicated to improve oxygenation and reduce the need for extracorporeal membrane oxygenation in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension in conjunction with ventilatory support and other appropriate agents. Refer to the full Prescribing Information within the LungFit PH System Operator’s Manual before use.
IMPORTANT SAFETY INFORMATION
- The nitric oxide from the LungFit PH is contraindicated in neonates dependent on right-to-left shunting of blood.
- Methemoglobin levels increase with the dose of nitric oxide; it can take 8 hours or more before steady-state methemoglobin levels are attained. Monitor methemoglobin and adjust the dose of nitric oxide to optimize oxygenation.
DEVICE WARNINGS
- Persons using the LungFit PH System should be trained and experienced in its use before performing procedures described in this manual to assure effective administration of nitric oxide and to avoid injury to the patient or to others resulting from inhalation of excess nitric oxide, nitrogen dioxide, or other reaction products.
- Abrupt discontinuation of nitric oxide may lead to worsening oxygenation and increasing pulmonary artery pressure, ie., Rebound Pulmonary Hypertension Syndrome. Signs and symptoms of Rebound Pulmonary Hypertension Syndrome include hypoxemia, systemic hypotension, bradycardia, and decreased cardiac output. If Rebound Pulmonary Hypertension occurs, reinstate nitric oxide therapy immediately.
- A non-expired NO2 Filter must be properly installed at all times for the LungFit PH System to function. Always have at least 1 spare NO2 Filter available.
- Do not make unauthorized changes to the device as it can result in harm to the patient.
CAUTION
- To maintain the LungFit PH batteries in a charged state, keep the system plugged in between uses.
Rx Only
Consult the LungFit PH System Operator’s Manual for complete information. For technical support, call 1-855-586-4359.



